774
Views & Citations10
Likes & Shares
Reaction between venom-antivenom molecule with receptor site is modeled here as a catalysis problem. Venoms, mostly rich in protein and peptide toxins, have specificities for a wide range of tissue receptors, making them clinically challenging and scientifically fascinating. In this article, venom-antivenom interaction mechanism is modeled kinetically in an attempt to elucidate the probable path including the concept of receptor site coverage. It explores the effect of on time and also the most common condition of delayed antivenom injection on receptor site and also provides guidance for optimum antivenom dose regime. Currently, mathematical and computer modeling are widely used to assess the behavior of complex systems. Literature data are fitted in kinetic schemes, starting from a very general route and gradually attain a sequential or random bi-substrate mechanism and subsequently to a further simplified Michaelis-Menten model. Besides, some interesting aspects of the problem are highlighted. A motif reduction technique is applied to the kinetic network achieving ample simplification. Pilot calculations on the snake venom kinetics show considerable promise.
Keywords: Venoim-antivenom kinetics, Antivenom dosage, Bisubstrate kinetics
INTRODUCTION
Poisonous snake bite may be life taking. Understanding snake venom pharmacokinetics is essential for developing risk assessment strategies and determining the optimal dose and timing of anti-venom required to bind all venom in snakebite patients. In 2009, WHO listed snakebite as a neglected tropical disease, recognizing its importance alongside many infectious diseases. This is a particularly important public health issue in tropical and subtropical regions mostly affecting those who live in rural areas, including the agricultural workforce in developing countries. Snake envenomation causes significant morbidity and mortality usually requiring hospitalisation and occasionally causing permanent disabilities and in severe cases death. Delayed accesses to appropriate medical facilities, lack of anti-venom and limited supportive treatments are considered the main contributors to the high morbidity and mortality. The only scientifically and medically approved treatment [1] of envenoming by snake especially is the use of intravenously administered anti-venom. But there is limited information on the pharmacokinetics and appropriate dosing regimen. Most of the research articles concentrate on either the isolation of chemicals from venom [2] in preparing specific drugs for neutralizing venom toxins or preparation of anti-venom, analyzing snake venom [3] components. Again most studies of the pharmacokinetics of anti-venom are in animals [4] and the pharmacokinetics appear to differ between animals, making animal models problematic for defining the pharmacokinetics of anti-venom in humans. Moreover heterogeneity in pharmacokinetics and mechanism of action of venom components require a detailed analysis of each venom-anti-venom system in order to determine their mode of kinetic route and their pharmacokinetic-pharmacodynamic relationship in order to optimize therapy of envenomation.
The major disadvantages of anti-venom therapy are: (a) Side effects to human body such as anaphylactic shock, pyrogen reaction, etc. Most of these symptoms may be due to the action of high concentrations of commercially available anti-venom; (b) Failure to neutralize the toxic components of the venom causing local hemorrhage, necrosis and tissue damage in snake bite victims; (c) Due to geographical variation in venom composition of snakes, anti-venom raised against the venom of a snake from a particular geographical origin may not be able to neutralize or prevent local effect of envenomation by snakes from other areas; (d) Scarcity of sufficient amount of quality venom and lack of storage facility in rural areas; (e) Due to the difficulty in identifying the snake species, instead of using monovalent type, polyvalent type anti-venom is commonly used, which may be hazardous to the patient and likely to be less effective. Dosing and assessment of the effectiveness of anti-venom in human envenoming remains controversial [1,4] and treatment protocols are not based on the kinetics of venom or anti-venom. In this backdrop, the major challenge to the scientific community is in understanding the time course of anti-venom in patients with snake envenoming, which will provide a better basis for anti-venom dosing.
The present article discusses the enumeration of plausible core mechanistic paths that any venom-anti-venom system follows so that appropriate drug measure may modulate the kinetics. This may also provide a thorough understanding of envenomation and the therapeutic remedial route, through design of experiments that may distinguish them.
THE SCHEME
The severity of the problem in understanding venom-anti-venom kinetics is due to the fact that there are several mechanisms of action for particular venom and toxicity of venom cannot be attributed to only one component. They are cocktails of enzymes and non-enzymatic protein toxins with diverse biological activities. This work is motivated by the kinetic scheme presented in the literature by Fogler [5] and Gurmen and Fogler [6] with detail rate constants for snake venom kinetics. This is referred to as sequence (1).
The differential equations governing the venom-anti-venom kinetic pathway and the reported rate constant values are detailed in the Appendix 1 for a thorough understanding of the equations presented in the main text. The rate constants for the individual paths are those from the article cited above [5,6]. The mass conservation equations that may be applied are given.
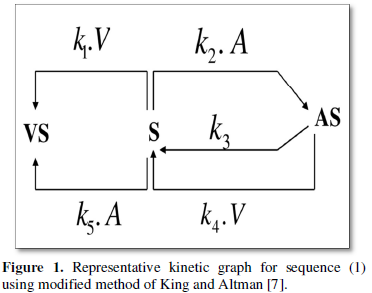
To easily derive the general rate equations, theory of kinetic graphs, a modified King-Altmans method [7-9] is exploited to avoid the cumbersome steady state calculation. For the given sequence (1a-1h) the representative kinetic graph is given by Figure 1 and the corresponding basic determinants of the vertices for Figure 1.
The reaction rate (r) using King-Altman method is given as follows [7]:
The above equation (4) is however too complex too to be used for the estimation of the kinetic constants. Several simplified models may be proposed here. The numerically simulated rate however validates the theoretically estimated rate form (4) which is another check on the theoretical endeavor.
MATERIALS AND METHODS
The kinetic pathway is simulated numerically over time. Since the equations are highly coupled and nonlinear, one needs to employ an accurate and robust method with a very small time step [10]. To this end, here we use the fourth-order Runge-Kutta method with a time step of order 10-4-10-5. A clear check of the stability of the simulation procedure is provided by the constancy of conservation equations (7) at each time step over the entire time-interval of interest.
On plausible mechanism of venom-anti-venom kinetics, it is now well established that anti-venom acts to neutralize venom and causes venom to be released from receptor site. The sites are then free to interact with chemicals (acetylcholine esterase) to resume respiration. Literature also suggests that with 80-90% of site blockage the victim is at a death risk. Most of articles use ‘twitch height’ as an important parameter to study and explain the pharmacokinetics of snake bite as a measure of muscular function and response [5,6]. Blocking of receptor site by venom inhibits muscular response which is measured by decrease in twitch height.
Based on the similar line of thought, fraction of free sites (f=s/s0) as a parameter is introduced in this work to deal with the pharmacological effect of the kinetics. It is suggested that respiration will be affected if f goes below a certain minimum value corresponding to maximum allowable site blocking for physiological functioning. This article also proposes a ‘therapeutic window time’ within which if anti-venom therapy is capable of reviving the blocked site, then chances of recovery of patient/victim is better. However, if site blockage continues beyond the window, no matter any amount of antivenin will not be able to sustain/restore the life of the victim (point of no return). Based on this, investigation is carried out on the following lines: (i) whether at all there is any optimum drug dose for envenomation; (ii) change in optimum dose if required when time lapse is different for different cases of envenomation and the point of time of drug administration; (iii) whether antivenin dosage may be altered by injecting little dose in parts over a period of time instead of a single full dose that often causes major side effects; (iv) plausible mechanistic route for a given venom-anti-venom system; (v) understanding of the core kinetic path to facilitate fitting of the parameters or to embed a detailed model of interest in a more coarse-grained yet realistic environment. The article thus considers the application of more objective method of anti-venom dosage therapy course during envenoming.
RESULTS AND DISCUSSION
On optimum dose of antivenin
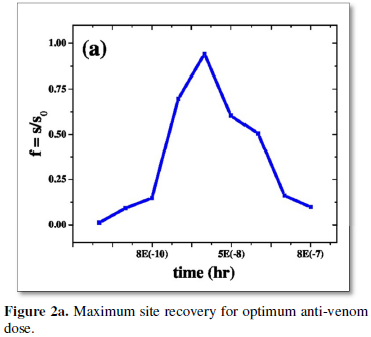
The dose and timing of anti-venom administration is still largely empirical and often based on in vitro neutralisation studies in animals, in which the toxic effects differ compared to humans. The dose of anti-venom [11,12] to be administered depends on the amount of the toxic proteins injected during a bite, in addition to the kinetics of venom distribution in the body. Therefore, one of the important requirements to develop improved anti-venins [1] and its suitable dose is the precise information of the quantity of venom ejected by a single bite. Again in particular, it remains unclear how long post-bite the administration of anti-venom remains an effective therapeutic intervention. A few observations from the simulation show that there is always an optimum antivenin dose for any envenomation when recovery of respiratory site is the maximum (Figure 2a).
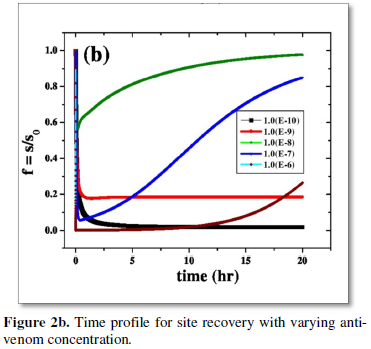
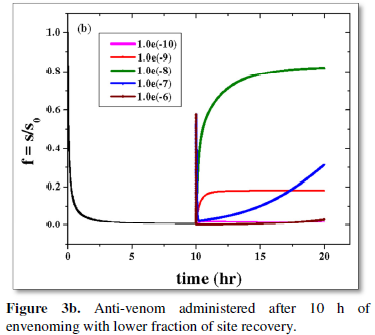
Several possible cases of envenoming is explored with no or late antivenin administration. Study includes cases where antivenin dosage is administered slowly over a period in parts, cases of ineffective dose or overdose of anti-venom and finally provides important information about the time course of the exposure to venom. First the case is explored where anti-venom is injected immediately after envenomation. It is observed that an ‘optimum dose’ of order 1.0 (e-8) can recover eighty percent of the respiratory site within a couple of hours, and dose lower than this is ineffective. It is also a caution to note that higher dose of antivenin 1.0 (e-7) requires a much longer time for essential site recovery which might be in some cases, outside the purview of ‘therapeutic window time’ as mentioned in the previous para. A much higher dose 1.0 (e-6) fails to recover breathing sites totally (Figure 2b), as they itself interact with sites disrupting their essential functions. Hence clinical practice of administering any arbitrary amount of antivenin dosage to a victim is thus strictly prohibited. The importance of providing guidance on initial optimum dosages is very much recommended. It is also suggested that anti-venom should be used only in patients in whom the benefits of treatment are considered to exceed the risks of anti-venom reactions [1].
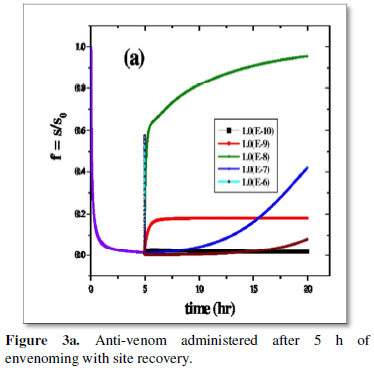
The basic observation is however same for anti-venom dose administered few hours post-bite (5 h in Figure 3a and 10 h for Figure 3b). However essential site revival is inversely proportional to passage of elapsed time between envenoming and drug administration is clear on comparison of the respective Figures 3a and 3b. The order of the dose regimen is however kept the same as in Figure 2b for a thorough comparative understanding. The health system needs to respond to this challenge.
On anti-venin dose regimen
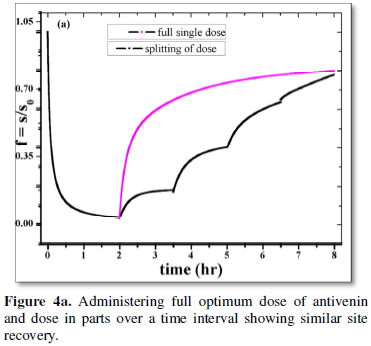
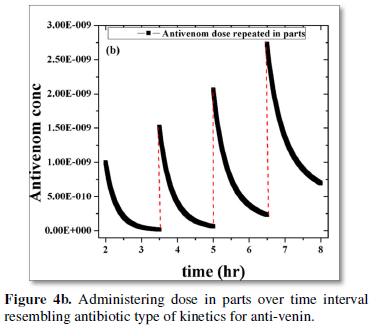
Other aspect of the problem is explored by comparison of site recovery through infusion of: (i) a constant full antivenin dose at a given time (Figure 4a); (ii) splitting the optimum dose in parts over a period of about 8-10 h with a time gap of a couple of hours as in case of antibiotic kinetics (Figures 4a and 4b). A motivating observation is that the site recovery is almost the same for both the instances. However the latter mode of anti-venom dosage may be embraced to minimize the dangerous local side-effects of anti-venom on patients [1,11-14].
PLAUSIBLE MECHANISTIC ROUTE
Bi-substrate kinetics
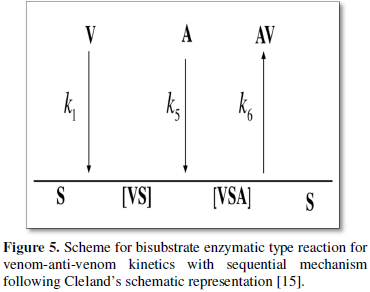
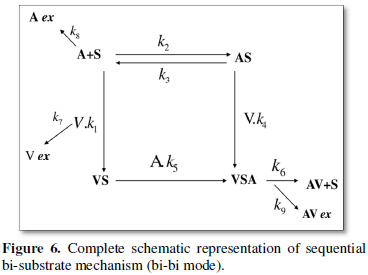
The venom-anti-venom interaction can be thought of as one in which there are two competing substrates that individually gives MM type kinetics when studied separately. But the physiological condition within the cellular system is mostly such that an enzyme has to choose between the substrates (enzyme specificity thus defined). A sequential bi-substrate type reaction pathway is thus proposed for the venom-anti-venom interaction. The basic scheme is given by Figure 5. According to Cleland’s representation [15], different states of enzyme (S, sites as in the present scheme) can be represented by horizontal line [8] and substrates along with products by vertical arrowhead lines. Venom (V) and anti-venom (A) as substrates, binary complex is given by (VS); (VSA) as ternary complex and (AV) as product respectively. The neutralization between venom-anti-venom occurs from the ternary complex. The full reaction scheme (1a-1h) is simulated with the complete bi-substrate kinetic route represented by Figure 6.
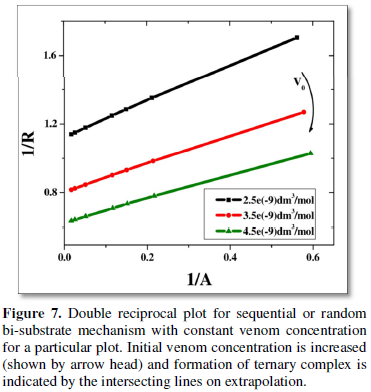
Double reciprocal type of plot [13,14] of 1/R (inverse of rate) versus 1/A (i.e., inverse of anti-venom concentration) is done from the simulated data varying the initial concentration of venom (V0).
The values of slope and intercepts obtained for the different plots are inversely related to the initial concentration of venom taken (Table 1). This justifies either sequential or random bi-substrate mechanism or the presence of a ternary complex.
The mechanistic pathway proposed via Figures 5 and 6, is pictorially simple but analytically difficult to track, as evident from any standard enzyme kinetics textbook. The equations may even be derived for the sequence but skipped to reduce the bulk from the article. Hence the model can be further simplified with reasonable assumptions.
Further Reduction to MM kinetics:
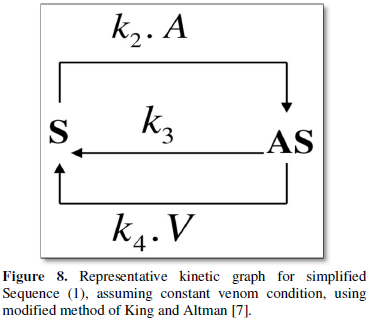
Binding mechanism of the snake venoms having catalytic activities [16,17], is expected to follow Michaelis-Menten (MM) type of kinetics. The equations and the corresponding ODE’s governing the venom-anti-venom interaction (Appendix 1) and the rate equation (4) from kinetic graph including the bi-substrate kinetics proposed are too complicated to intuit and unwieldy to give physical insights to the kinetic problem. Hence the analysis is simplified with the assumption of constant venom concentration within a victim’s body for a particular bite. The representative sequence through kinetic graph is thus reduced to Figure 8.
The moderate determinants for the vertices in Figure 8 of the kinetic graph representation are now:
The reduced reaction rate
The rate
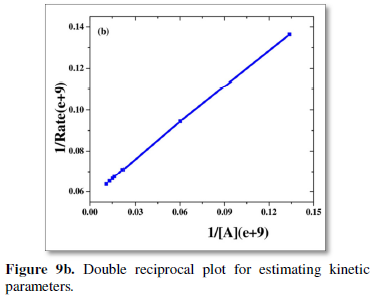
Equation for the double reciprocal plot (Figure 9b) of (6) that estimates a few rate constants or a bundle of them together is given by:
Where
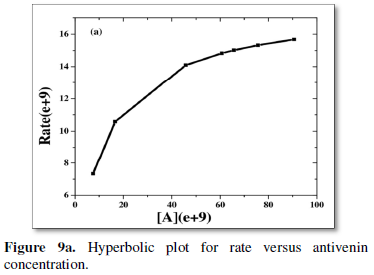
Simulated data used for plotting Figure 9b is tabulated. The intercept value estimated from the plot is 0.0577 (e+9), from which rate constant k4 is estimated as 6.9 (e+8) that closely corresponds to the reported value of 6.0(e+8). Ratio of (k3/k2) assessed form the slope (0.592) is 1.03 (e+8) near to 0.5 (e+8) of reported data [5,6].
Thus the set of equations (1a-h) can be reduced to a MM type of simplified scheme:
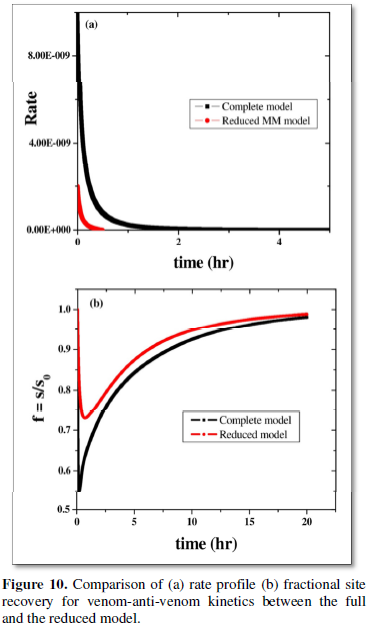
The number of dynamical equations for the network sequence 1 is thus reduced in such a way that the behavior of the key metabolites is approximated in a satisfactory way via (8). This is shown by plotting rate as well as site recovery parameter with time for the complete network and the reduced model (Figures 10a and 10b).
The model reduction strategy may be justified by adding the sub reaction sets (1a-1h) of sequence 1, that gives the final stoichiometric reaction as:
This reaction is however already included in the sub reactions provided as (1e). It is interesting to note that excluding the elimination reactions (1f-1h) and sub-reaction (1e), addition of equations (1a-1d) again gives the stoichiometric reaction as:
Finally, the reduced simplified scheme (8) that resembles enzymatic MM equation gives:
as the main stoichiometric equation, that rationalizes the model reduction to (8). The site (S) which is recovered on anti-venom injection, thus plays the part of enzyme, has been confirmed from its temporal profile. Venom-anti-venom complex (AV) as the product and anti-venom (A) as the substrate rationalize MM kinetics. Rate and site recovery, as parameters, for both the complete model as well as the reduced one are plotted (Figures 10a and 10b). Resemblance in their qualitative nature justifies the worth of the motif reduction strategy.
CONCLUSION
To summarize, a network governing venom-anti-venom kinetics is numerically and theoretically analysed to get new physical insights into the problem of venom-anti-venom interaction. The upshot of the article is two-fold: (a) to highlight a few interesting observations; and (b) to achieve a network reduction. The findings of the simulation recommend a few cautions in the form of observations: (i) For every envenoming, there is an initial optimum anti-venom dose that needs to be identified before its administration on victims (Figure 2a). (ii) Apart from the choice of antivenin, the dose interval, frequency and duration of administration must be included in a treatment protocol; otherwise, an arbitrary initial dose may prove to be fatal for victims (Figure 2b). (iii) A safe alternative is to repeat a small dose of anti-venom in over a time span of hours (Figures 4a and 4b). (iv) The dosage depends also on the time lapse between envenoming and treatment (Figures 3a and 3b).
On the reduction strategy, the network is justified in terms of bi-substrate kinetics. The set of nine dynamic equations are trim down to three in the facet of MM network. Thus, deciding a simplified kinetic pathway will help in estimating the kinetic parameters KM and kcat that may be very useful for the study and comparison of different site recovery in relation to anti-venin concentration. These parameter values reflect the cellular environment, substrate concentration, and chemical characteristics of the reaction catalyzed and an indicator of the affinity of the site for the anti-venom.
Thus increased collaboration between clinicians, epidemiologists and laboratory toxicologists should enhance the understanding and treatment of envenoming [18-21]. It is intended that the information provided in this review will provide a useful basis for future studies that address the pharmacokinetics of snakebite in humans. The article will probably contribute positively towards that end.
ACKNOWLEDGEMENT
The author acknowledges University Grants Commission, India for a research fellowship in the form of Dr. D. S. Kothari Post-doctoral fellowship and thanks Prof. K. Bhattacharyya for valuable discussions and constructive comments.
- Warrell DA (2010) Guidelines for the management of snakebite. World health Organization, USA.
- Izidoro LFM, Sobrinho JC, Mendes MM, Costa TR, Grabner AN, et al. (2014) Snake venom L-amino acid oxidases: Trends in pharmacology and biochemistry. BioMed Res Int 2014: 19.
- Amel KS, Fatima LD (2015) Purification and characterization of a new serine protease (VLCII) isolated from Vipera lebetina venom: Its role in hemostasis. J Biochem Mol Toxicol 29: 388-397.
- Yap MKK, Tan NH, Sim SM, Fung SY, Tan CH (2015) The effect of a polyvalent anti-venom on the serum venom antigen levels of Naja sputatrix (Javan spitting cobra) venom in experimentally envenomed rabbits. Basic Clin Pharmacol Toxicol 117: 274-279.
- Fogler HS (1999) Elements of chemical research engineering. 3rd Edn. Prentice-Hall, Inc.
- Gurmen NM, Fogler HS (2004) Web modules in the emerging areas of chemical reaction engineering. Proceedings of the 2004 American Society for Engineering Education Annual Conference and Exposition, pp: 2153-5965.
- King EI, Altman C (1956) A schematic method of deriving the rate laws for enzyme-catalyzed reactions. J Phys Chem 60: 1375-1378.
- Raducan A, Oancea D, Segal EI (2007) Models for irreversible inactivation in the enzymatic decomposition of hydrogen peroxide and their kinetic treatment by means of graphs. Revue Roumaine de Chimie 52: 713-717.
- Segal EI (2009) Applications of an algorithm to derive rate equations by means of kinetic graph. Revue Roumaine de Chimie 54: 719-722.
- Press WH, Teukolsky SA, Vetterling WT, Flannery BP (1992) Numerical recipes in FORTRAN 77. In: The Art of Scientific Computing. 2nd Edn. New York: University of Cambridge.
- Theakston RDG (1995) The kinetics of snake bite envenoming and therapy. Journal of the Ceylon College of Physicians 28: 42-45.
- Theakston RDG, Laing GD (2014) Diagnosis of snakebite and the importance of immunological tests in venom research. Toxins 6: 1667-1695.
- Mitra J, Sharma K, Bhattacharyya D (2015) Molecular modeling approaches for designing inhibitors of L-amino acid oxidase from Crotalus adamenteus venom. Curr Sci 108: 1086-1096.
- Mitra J, Bhattacharyya D (2013) Irreversible inactivation of snake venom L-amino acid oxidase by covalent modification during catalysis of L-propargylglycine. FEBS Open Bio 3: 135-143.
- Cleland WW (1963) The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta 67: 104-137.
- Gutierrez JM, Leon G, Lomonte B (2003) Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin Pharmacokinetics 42: 721-741.
- Riviere G, Choumet V, Audebert F, Sabouraud A, Debray M, et al. (1997) Effect of anti-venom on venom pharmacokinetics in experimentally envenomed rabbits: Toward an optimization of anti-venom therapy. J Pharmacol Exp Ther 281: 1-8.
- Dhatt S, Bhattacharyya K (2013) Enzyme kinetics: A critique of the quasi-steady state approximation. Commun Math Comput Chem 70: 759-84.
- Jalali A, Moazen S, Babaee M, Dadashzade S, Droudi A (2010) The pharmacokinetics of Iranian scorpion Odonthubuthus doriae venom and the available anti-venom. J Venom Res 1: 48-53.
- Ahmed W, Ahmad M, Khan RA, Mustaq N (2016) Promising inhibition of krait snake’s venom cetylcholinesterase by Salix nigra and its role as anticancer, antioxidant agent. Indian J Anim Res 50: 317-323.
- Isbister GK, Maduwge K, Salao A, Buckley NA, Jayamanne SF, et al. (2015) Population pharmacokinetics of an Indian F(ab')2 snake anti-venom in patients with Russell's viper (Daboia russelii) bites. PLoS Negl Trop Dis 9: e0003873.